Monoclonal Antibodies

Our new class of monoclonal antibodies is termed Variable Lymphocyte Receptors, or more simply VLRs. VLRs are the immune receptors of jawless vertebrates (lamprey and hagfish), are composed of a variable number of tandemly linked highly diverse leucine-rich repeat (LRR) domains and are the only known antigen-specific immune receptors that are not immunoglobulins (Igs). The VLR binding site is entirely contained within a single polypeptide and is a rigid beta-sheet structure that forms the VLR concave surface (see Figure). The over 500 million years that separate jawless vertebrates from humans and other mammals, and the distinctive binding site structure of VLR antibodies have enabled the discovery of specificities distinct from those of conventional Ig antibodies. The VLR single polypeptide structure enables development of novel functions, including highly multivalent antibody polymers and multispecific antibodies.
Ribbon diagram of a VLR antibody that is composed of LRR1, 4 LRRVS, N-terminal capping LRRNT and C-terminal capping LRRCT with a helical connecting peptide, CP, linking LRRVe to LRRCT. All VLRs consist of LRRNT, LRR1, CP, LRRCT and from 1 (LRRVe) up to 7 LRRV domains. The binding site is the concave surface.
We are able to “weaponize” VLR monoclonal antibodies to execute the immune effector functions of conventional Ig antibodies and immune receptors by genetically replacing the antigen recognition domains, “variable regions”, of Ig antibodies and receptors with VLRs (see Figure). These VLR-Ig “chimeras” link the effector function(s) of Ig antibodies and cellular receptors with the novel antigen recognition specificities of VLRs and capitalize on the ease with which VLRs enable multivalency and multispecificty to be incorporated. VLR-Ig chimeras are produced with the same processes developed for manufacturing Ig monoclonal antibody therapies and Ig-based chimeric antigen receptors (“CARs”), are administered to patients with the same formulations and regimens used for conventional Ig-based monoclonal antibody therapies, and are expected to demonstrate the well-characterized biodistribution and pharmacokinetic properties of conventional Ig monoclonal antibody therapeutics.
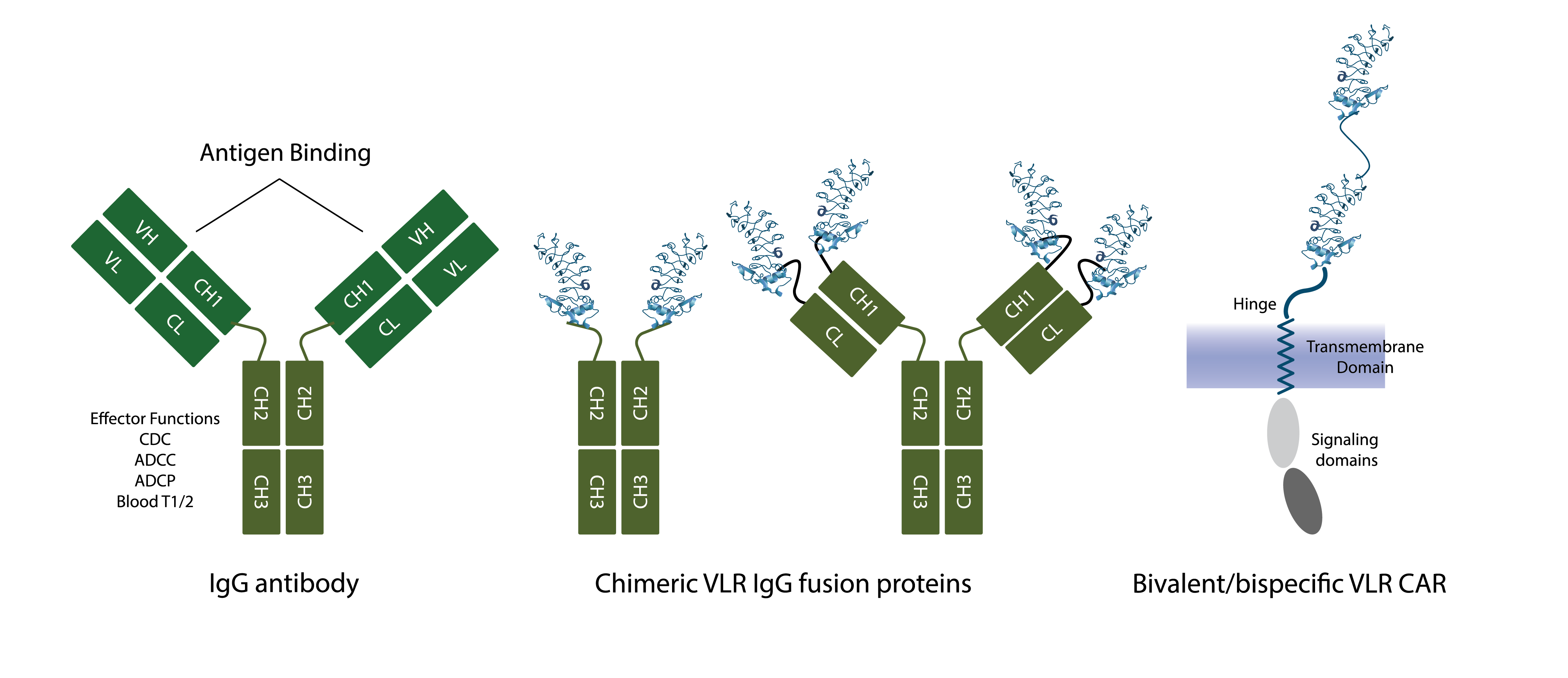
Note that the VLRs may be the same specificity, e.g., tetravalent VLR IgG chimera vs bivalent IgG antibody, or different specificities, e.g., bivalent/bispecific VLR IgG chimera. The IgG constant regions that mediate immune effector functions are maintained in the VLR IgG chimeric structures and provide immune effector function and extended blood half-life.
The surfaces of all cells and viruses are highly multivalent with respect to proteins and glycans, i.e., each distinct surface protein and glycan is typically present in thousands of identical copies on cell surfaces and hundreds of identical copies for viral surfaces. These multivalent “targets” are ideal for essentially irreversible binding by NovAb’s VLR antibody polymer reagents (see Figure). As illustrated, the envelop of SARS-CoV-2 is composed of a highly multivalent array of the Spike (S) protein and is a typical example of a multivalent target for binding by VLR polymer reagents. The VLRs that make up the VLR polymer may all have the same specificity (“monospecific”) and bind to the same target on the surface of cells or viruses or have distinct specificities (“multispecific”) and bind to distinct surface expressed targets or distinct epitopes of the same target. Like CoV-2 the surfaces of all viruses and all cells present highly multivalent targets for high sensitivity detection with multivalent VLRB polymers. We plan to develop VLR polymer reagents for detecting other infectious agents and for detecting aberrant cells, e.g., tumors, as well.
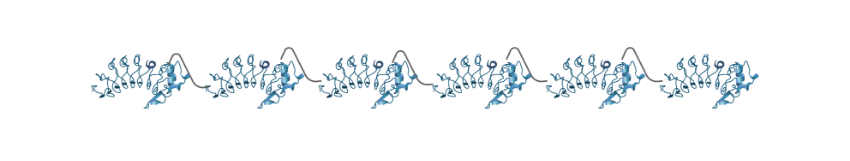
VLR polymers are composed of tandemly linked VLRS
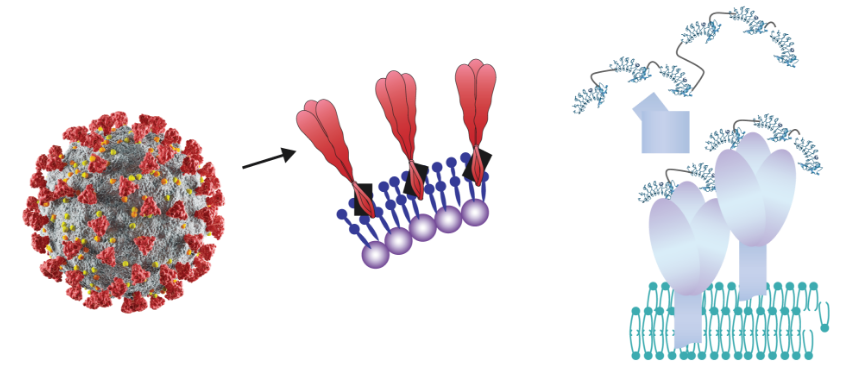
The envelop of SARS-CoV-2 virus comprises a large number of “Spikes”. Each Spike is composed of three identical copies of the same Spike protein.
NovAb is addressing an important unmet need within the biomedical research community for development and commercialization of highly specific reagents that bind and discriminate glycans. Glycans are composed of simple sugars (monosaccharides) linked together to form larger and more complex oligosaccharides and yet larger polysaccharides. Glycans are abundantly expressed on the surfaces of all cells and viruses, are more abundant than cell surface proteins, and like proteins, glycans mediate critical functions including cell migration and signaling, interactions with bacterial and viral pathogens, and regulation of both innate and adaptive immunity.

Glycans on the surfaces of cells, including bacteria and viruses, interact with cell surface glycan binding proteins to mediate diverse and important functions, including cell migration and adhesion, viral and bacterial infection, immune regulation, tumor formation and metastasis. Interruption of these interactions with glycan-specific VLR antibody reagents promises to reveal significant new biology with therapeutic potential.
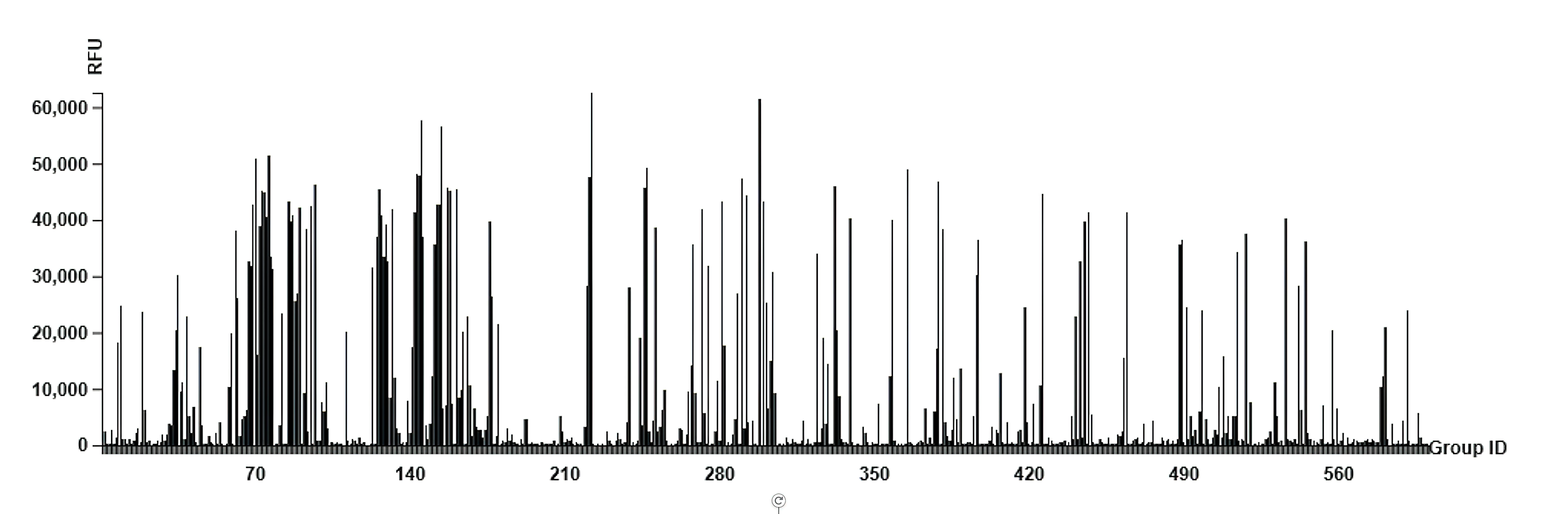
Analysis of serum from lamprey immunized with a human cancer cell line using the Consortium for Functional Glycomics (CFG) version 5.1 glycan array. The array contains over 600 distinct compositions of glycan linker structures. Each vertical line indicates the presence in the serum sample of a VLR antibody that binds the glycan present at that location in the array and the height of the vertical lines is an indication of both the strength of binding and relative abundance of VLR antibodies that bind that glycan.
